1. Jelaskan dalam jalur biosintesis
triterpenoid, identifikasilah faktor-faktor penting yang sangat menentukan
dihasilkannya triterpenoid dalam kuantitas yang banyak.
Mechanism:
isopentyl pyrophosphate produced from acetyl-CoA to mevalonic acid pathway to
form dimethyl terisomerisasi alipirofosfat with the help of an enzyme
(isomerase) makes merging them to produce geranil pyrophosphate, while the
compound is re-joining the isopentyl pyrophosphate to yield pyrophosphate compound
famesil as sesquiterpenoid compounds, then track the same mechanism occurs so
both famesil pyrophosphate combine to produce compounds triterpenoids. Having
regard to the condition of these biosynthetic pathways can be changed in order
to produce more terpenoids. But there are some factors that really determine
the biosynthesis of these, namely:
1.
Enzyme levels to pay attention to the optimization of these enzymes work.
2.
Method of isolation.
3.
Kontrolisasi against acid pathway so as not to inhibit other pathways in the
biosynthesis of this.
4.
Environment that does not interfere with the ongoing process of biosynthesis of
these.
2. Jelaskan dalam penentuan struktur flavonoid,
kekhasan signal dan intensitas serapan dengan menggunakan spektrum IR dan NMR.
Berikan dengan contoh sekurang-kurangnya dua struktur yang berbeda.
Infrared Spectroscopy (IR)
Compared to the wavelength of ultraviolet light and visible, infrared wavelengths are longer and thus lower energy. Energy infrared light is related to the vibrational energy of molecules. Excited molecule would correspond to wavelengths absorbed. Stretching vibration and bending vibration is the way to diekstitasi by rays with gelombag number (the number of waves per unit length) in the range of 1200-4000 cm-1.
How to read the FTIR spectra:
1. Determine the X-axis and Y-axis of the spectrum. X-axis of the IR spectrum are labeled as "wave numbers" and the numbers range from 400 to 4,000 in the far right on the far left. X-axis provides the number of absorption. Y axis is labeled as "Percent transmittance" and the numbers range from 0 at the bottom and 100 on top.
2. Determine the characteristic peaks in the IR spectrum. All the infrared spectrum contains many peaks. Furthermore, looking at the data area of functional groups required for reading spectrum.
3. Determine the area of the spectrum where there are characteristic peaks. IR spectra can be separated into four regions. The first area ranges from 4,000 to 2,500. The second region ranges from 2,500 to 2,000. All three areas ranged from 2,000 to 1,500. The fourth area ranges from 1,500 to 400.
4. Determine the functional groups absorbed in the first area. If the spectrum has a characteristic peak in the range of 4,000 to 2,500, according to the absorption peak due to NH, CH and OH single bond.
5. Determine the functional groups absorbed in the second. If the spectrum has a characteristic peak in the range of 2,500 to 2,000, according to the absorption peak caused by a triple bond.
6. Determine the functional groups absorbed in the third. If the spectrum has a characteristic peak in the range of 2,000 to 1,500, according to the absorption peak caused by the double bonds such as C = O, C = N and C = C.
7. Compare peak in the fourth to peak in the fourth IR spectra others. The fourth is known as the fingerprint region of the IR spectrum and contains a large number of absorption peaks that account for a wide range of single bonds. If all the peaks in the IR spectrum, including those in the fourth, is identical to the peak of the spectrum, then you can be sure that the two compounds are identical.
Table area of functional groups on IR:
Compared to the wavelength of ultraviolet light and visible, infrared wavelengths are longer and thus lower energy. Energy infrared light is related to the vibrational energy of molecules. Excited molecule would correspond to wavelengths absorbed. Stretching vibration and bending vibration is the way to diekstitasi by rays with gelombag number (the number of waves per unit length) in the range of 1200-4000 cm-1.
How to read the FTIR spectra:
1. Determine the X-axis and Y-axis of the spectrum. X-axis of the IR spectrum are labeled as "wave numbers" and the numbers range from 400 to 4,000 in the far right on the far left. X-axis provides the number of absorption. Y axis is labeled as "Percent transmittance" and the numbers range from 0 at the bottom and 100 on top.
2. Determine the characteristic peaks in the IR spectrum. All the infrared spectrum contains many peaks. Furthermore, looking at the data area of functional groups required for reading spectrum.
3. Determine the area of the spectrum where there are characteristic peaks. IR spectra can be separated into four regions. The first area ranges from 4,000 to 2,500. The second region ranges from 2,500 to 2,000. All three areas ranged from 2,000 to 1,500. The fourth area ranges from 1,500 to 400.
4. Determine the functional groups absorbed in the first area. If the spectrum has a characteristic peak in the range of 4,000 to 2,500, according to the absorption peak due to NH, CH and OH single bond.
5. Determine the functional groups absorbed in the second. If the spectrum has a characteristic peak in the range of 2,500 to 2,000, according to the absorption peak caused by a triple bond.
6. Determine the functional groups absorbed in the third. If the spectrum has a characteristic peak in the range of 2,000 to 1,500, according to the absorption peak caused by the double bonds such as C = O, C = N and C = C.
7. Compare peak in the fourth to peak in the fourth IR spectra others. The fourth is known as the fingerprint region of the IR spectrum and contains a large number of absorption peaks that account for a wide range of single bonds. If all the peaks in the IR spectrum, including those in the fourth, is identical to the peak of the spectrum, then you can be sure that the two compounds are identical.
Table area of functional groups on IR:
NMR spectroscopy
Many of the core (or more precisely, the core with the least odd number of protons or neutrons) can be considered as a small magnet. Core like proton (1 H or H-1) and carbon-13 nuclei (13C or C-13; approximately 1% natural abundance). Carbon -12 (12C), which is used as a standard determination of mass, not magnetic.
When a sample containing 1H or 13C (even all organic compounds) is placed in a magnetic field, there will be interaction between the external magnetic field with a magnet was small (nucleus). Because there is this interaction, a small magnet will be divided into two energy levels (levels a little bit more stable (+) and conditions that are less stabel (-)) a different energy. Because the world is a world of microscopic nuclei.
Many of the core (or more precisely, the core with the least odd number of protons or neutrons) can be considered as a small magnet. Core like proton (1 H or H-1) and carbon-13 nuclei (13C or C-13; approximately 1% natural abundance). Carbon -12 (12C), which is used as a standard determination of mass, not magnetic.
When a sample containing 1H or 13C (even all organic compounds) is placed in a magnetic field, there will be interaction between the external magnetic field with a magnet was small (nucleus). Because there is this interaction, a small magnet will be divided into two energy levels (levels a little bit more stable (+) and conditions that are less stabel (-)) a different energy. Because the world is a world of microscopic nuclei.
A table of typical
chemical shifts in C-13 NMR spectra
carbon environment
|
chemical shift (ppm)
|
C=O (in ketones)
|
205 - 220
|
C=O (in aldehydes)
|
190 - 200
|
C=O (in acids and
esters)
|
170 - 185
|
C in aromatic rings
|
125 - 150
|
C=C (in alkenes)
|
115 - 140
|
RCH2OH
|
50 - 65
|
RCH2Cl
|
40 - 45
|
RCH2NH2
|
37 - 45
|
R3CH
|
25 - 35
|
CH3CO-
|
20 - 30
|
R2CH2
|
16 - 25
|
RCH3
|
10 - 15
|
example:
1. Quarcetin
1. Quarcetin
IR Spectrum
NMR
Spectrum
1. Carotenoid
IR Spectrum
Spectrum NMR
3. Dalam isolasi alkaloid, pada tahap
awal dibutuhkan kondisi asam atau basa. Jelaskan dasar penggunaan reagen
tersebut, dan berikan contohnya sekurang-kurangnya tiga macam alkaloid.
Alkaloids are
usually isolated from the plant by extraction method. Solvents
are used when extracting the compound mixture is acidified water molecules. This
solvent will be able to dissolve the alkaloid salts. Moreover,
it can alkalinize alkaloid-containing plant material by adding sodium
carbonate. bases
formed can then be extracted with organic solvents chloroform or ether.
For alkaloids that are not heat resistant, insulation can be done using techniques alkalinize the solution concentration by first, with this technique the alkaline alkaloid will evaporate and then be purified by steam distillation method. Solution in water that is acidic and contains alkaloids and alkaloid diekstaksi can basified with an organic solvent, so that neutral and acidic compounds are readily soluble in water left in the water.
So acidic and basic conditions needed one in the isolation of alkaloids due to the acidic conditions of the alkaloid as the salt will dissolve. As for the alkaloids that are not heat resistant to alkalinize the solution should dipekatan
Isolation of nicotine from tobacco leaves
The sample used is 100 grams so the extraction is done 4 times. Extract / filtrate resulting solution is evaporated until the resulting filtrate concentrated or only 10% of the original volume
Concentrated solution is poured into the erlenmeyer flask and acidified with 2 M H2SO4 at 25 mL. The solution was stirred with a magnetic stirer to be homogeneous. Then the solution was extracted with chloroform 25 mL 3 times a separating funnel. The resulting extracts were tested with reagents undercoat Dragendorf, if there is a positive alkaloid orange precipitate.
The extract was neutralized again by adding NH4OH and then extracted again with 25 mL of chloroform 3 times. The extract obtained was evaporated to aerate, then purified by column chromatography on silica gel 11.5 grams as the stationary phase, column length 10 cm, 3 cm diameter column and eluent n hexane and chloroform, methanol with a ratio of 1:0, 7:3 , 5:5, 3:7 and 0:1 respectively - each as much as 10 mL.
Example: Isolation of alkaloids from plant roots Anamirta cocculus (L.) W. & A. (Tuba seeds)
For alkaloids that are not heat resistant, insulation can be done using techniques alkalinize the solution concentration by first, with this technique the alkaline alkaloid will evaporate and then be purified by steam distillation method. Solution in water that is acidic and contains alkaloids and alkaloid diekstaksi can basified with an organic solvent, so that neutral and acidic compounds are readily soluble in water left in the water.
So acidic and basic conditions needed one in the isolation of alkaloids due to the acidic conditions of the alkaloid as the salt will dissolve. As for the alkaloids that are not heat resistant to alkalinize the solution should dipekatan
Isolation of nicotine from tobacco leaves
The sample used is 100 grams so the extraction is done 4 times. Extract / filtrate resulting solution is evaporated until the resulting filtrate concentrated or only 10% of the original volume
Concentrated solution is poured into the erlenmeyer flask and acidified with 2 M H2SO4 at 25 mL. The solution was stirred with a magnetic stirer to be homogeneous. Then the solution was extracted with chloroform 25 mL 3 times a separating funnel. The resulting extracts were tested with reagents undercoat Dragendorf, if there is a positive alkaloid orange precipitate.
The extract was neutralized again by adding NH4OH and then extracted again with 25 mL of chloroform 3 times. The extract obtained was evaporated to aerate, then purified by column chromatography on silica gel 11.5 grams as the stationary phase, column length 10 cm, 3 cm diameter column and eluent n hexane and chloroform, methanol with a ratio of 1:0, 7:3 , 5:5, 3:7 and 0:1 respectively - each as much as 10 mL.
Example: Isolation of alkaloids from plant roots Anamirta cocculus (L.) W. & A. (Tuba seeds)
4. Jelaskan keterkaitan diantara
biosintesis, metode isolasi dan penentuan struktur senyawa bahan alam . Berikan
contohnya.
Ø Biosynthesis
It is a process of formation of the compound or structure
Isolation of Natural Compounds Chemistry of Materials
Basically isolation of chemical compounds from natural ingredients it is a business how to separate the compounds were mixed so that we can produce a pure single senayawa. Let's take just one example, how to isolate compounds from plants. Plants that contain thousands of compounds, both of which are categorized as primary metabolites or secondary metabolites. Usually the isolation of compounds from natural materials, hopes to isolate the secondary metabolites, secondary metabolites due believed and has been shown to provide benefits for human life.
methods of insulating compound of natural ingredients extraction method is the process of decision-soluble components of the substance or mixture using a solvent such as water, alcohol, ether, acetone and so on. The extraction method was chosen to obtain a compound of natural materials depends on the type of plant samples and the type of compounds that exist. mainly depends on the physical state of these compounds, such compounds volatile liquid
can be classified as physical isolation and insulation means chemical means.
1. Physical Isolation Method
Isolation in this way by the physical properties of natural materials, such as solubility and vapor pressure. Isolation based on differences in solubility of certain natural substances in the solvent can be done with a cold solvent or solvent heat. Insulation with cold solvent used to isolate natural materials that can be dissolved in the cold. The technique can be done by soaking the natural source material in a specific solvent for some time (hours or days). For a natural material that dissolves in hot conditions used isolation techniques continuously by means of Soxhlet. Isolation based on vapor pressure reduction is done by steam distillation. This method is used for compounds that are not water soluble dalarn, high boiling, easy to decompose before boiling and volatile.
2. Isolation In Chemistry
Isolation way based on the chemical properties or reactivity of natural products against specific reagents. Natural materials were isolated through a chemical reaction and separated from other compounds that do not react.
It is a process of formation of the compound or structure
Isolation of Natural Compounds Chemistry of Materials
Basically isolation of chemical compounds from natural ingredients it is a business how to separate the compounds were mixed so that we can produce a pure single senayawa. Let's take just one example, how to isolate compounds from plants. Plants that contain thousands of compounds, both of which are categorized as primary metabolites or secondary metabolites. Usually the isolation of compounds from natural materials, hopes to isolate the secondary metabolites, secondary metabolites due believed and has been shown to provide benefits for human life.
methods of insulating compound of natural ingredients extraction method is the process of decision-soluble components of the substance or mixture using a solvent such as water, alcohol, ether, acetone and so on. The extraction method was chosen to obtain a compound of natural materials depends on the type of plant samples and the type of compounds that exist. mainly depends on the physical state of these compounds, such compounds volatile liquid
can be classified as physical isolation and insulation means chemical means.
1. Physical Isolation Method
Isolation in this way by the physical properties of natural materials, such as solubility and vapor pressure. Isolation based on differences in solubility of certain natural substances in the solvent can be done with a cold solvent or solvent heat. Insulation with cold solvent used to isolate natural materials that can be dissolved in the cold. The technique can be done by soaking the natural source material in a specific solvent for some time (hours or days). For a natural material that dissolves in hot conditions used isolation techniques continuously by means of Soxhlet. Isolation based on vapor pressure reduction is done by steam distillation. This method is used for compounds that are not water soluble dalarn, high boiling, easy to decompose before boiling and volatile.
2. Isolation In Chemistry
Isolation way based on the chemical properties or reactivity of natural products against specific reagents. Natural materials were isolated through a chemical reaction and separated from other compounds that do not react.
Ø Perform extraction using organic
solvents.
Ø Conduct chromatographic separation
with different methods such as using the partition method, chromatography
columns, planar chromatography, radial chromatography, HPLC etc.
Ø structure elucidation of compounds
that have been isolated by using various methods such as Infrared
Spectroscopies, spectrum of mass, NMR, etc.
Ø test them pharmacological activity
of compounds that have been isolated
Stage Compound Identification Results Isolation
Determination of molecular structure
by sticher in molecular structure determination obtained through spectroscopic
measurement steps for spectral data. After obtaining the spectral data of new
molecular structures determined.
The measures in question
as follows:
1. To measure the UV-Vis spectra to
determine the chromophore groups
2. To measure the IR spectrum for
the presence of functional groups
3. To measure the mass spectra (MS)
to determine the pattern of fragmentation
compounds.
Structure elucidation of organic
molecules can be done using spectroscopy methods with the instruments used are:
spectrophotometer ultraviolet (UV), infrared (IR), mass (MS), Nuclear Magnethic
resonance (13C-NMR, 1HNMR), Distortionless Enhancement by Polarization Transfer
(DEPT ), 1H-13C Heteronuclear Multiple Quantum Coherence (HMQC), 1 H-1 H
Homonuclear Correlated Spectroscopy (COSY) and 1H-13C Heteronuclear Multiple
Bond 20 Connectivity (HMBC) can follow the methodology as chart Santoni (2009)
as follows:
Ultraviolet Spectroscopy
For the purposes of determining the structure, ultra violet spectroscopy has the ability to measure the number of double bonds or aromatic conjugation in the molecule. Wavelength region of the UV spectrum range 200-400 nm. The absorption of ultraviolet light by a molecule will generate transitions between electronic energy levels of the molecule. The transition occurs at orbital bonding or lone pair with the anti-bonding orbital. System (groups of atoms) that causes the absorption of light are called chromophores.
Infrared Spectroscopy
Infrared spectrophotometry is more widely used for identification of a compound through the group functions. For the purpose of structure elucidation, the wavenumber region 1400 - 4000 cm-1 which is at the left of the IR spectrum, an area that is particularly useful for the identification of clusters.
1H-NMR Spectroscopy
1H-NMR spectroscopy is widely used by organic chemists. Spectroscopy is based on the fact that each group of protons (H) in organic molecules to resonate at a frequency that is not identical or resonate at specific frequencies. This is due to the proton electron surrounded by an organic molecule is different (different electronic environments). The greater the density of electrons surrounding the nucleus the greater the magnetic field used. Because each atom H (proton) an organic molecule has the electronic environment (chemical) is different, it will cause a different resonance frequencies (Sitorus, 2009). Chemical shift, denoted by δ, stating how far (ppm) proton is shifted from standard proton Tetrametilsilana (TMS)
Carbon NMR spectroscopy (13C-NMR)
Proton or 1H spectroscopy provides an overview of hydrogen atoms in an organic molecule. Spectroscopy of carbon-13 or carbon-13C provides an overview of carbon in an organic molecule. The spectra of carbon-13 is not used widely as proton spectra. Involved in proton spectroscopy is a common and natural isotope of hydrogen, a hydrogen atom is 99.985% 1H. But carbon-13 is only 1.1% of the carbon atoms are present in nature, because 98.9% of carbon atoms are 12C, a nucleotide that has no spin. 13C nuclei transition from parallel to antiparallel state is low-energy transition. Because of its abundance in nature is only 1.1%, the sensitivity of the 13C-NMR is much smaller than 1H which has 99.98% abundance in nature.
I think the relationship between biosynthesis, methods of isolation and structural determination of compounds from the natural ingredients are obtained form the compound biosynthesis of natural products that will be made in reference to identify the structure, the structure resulting from the process of biosynthesis of the structure would be similar to the results of the identification or determination of the structure of the compound natural ingredients, and after learning biosynthesis, isolation can do in order to separate the mixed compound so that we can produce a pure single senayawa. From the results of the isolation of a pure single compound we can determine the structure of these compounds using spectroscopic methods with the instruments used are: spectrophotometer ultraviolet (UV), infrared (IR), mass (MS), Nuclear Magnethic resonance (13C-NMR, 1HNMR ).
For the purposes of determining the structure, ultra violet spectroscopy has the ability to measure the number of double bonds or aromatic conjugation in the molecule. Wavelength region of the UV spectrum range 200-400 nm. The absorption of ultraviolet light by a molecule will generate transitions between electronic energy levels of the molecule. The transition occurs at orbital bonding or lone pair with the anti-bonding orbital. System (groups of atoms) that causes the absorption of light are called chromophores.
Infrared Spectroscopy
Infrared spectrophotometry is more widely used for identification of a compound through the group functions. For the purpose of structure elucidation, the wavenumber region 1400 - 4000 cm-1 which is at the left of the IR spectrum, an area that is particularly useful for the identification of clusters.
1H-NMR Spectroscopy
1H-NMR spectroscopy is widely used by organic chemists. Spectroscopy is based on the fact that each group of protons (H) in organic molecules to resonate at a frequency that is not identical or resonate at specific frequencies. This is due to the proton electron surrounded by an organic molecule is different (different electronic environments). The greater the density of electrons surrounding the nucleus the greater the magnetic field used. Because each atom H (proton) an organic molecule has the electronic environment (chemical) is different, it will cause a different resonance frequencies (Sitorus, 2009). Chemical shift, denoted by δ, stating how far (ppm) proton is shifted from standard proton Tetrametilsilana (TMS)
Carbon NMR spectroscopy (13C-NMR)
Proton or 1H spectroscopy provides an overview of hydrogen atoms in an organic molecule. Spectroscopy of carbon-13 or carbon-13C provides an overview of carbon in an organic molecule. The spectra of carbon-13 is not used widely as proton spectra. Involved in proton spectroscopy is a common and natural isotope of hydrogen, a hydrogen atom is 99.985% 1H. But carbon-13 is only 1.1% of the carbon atoms are present in nature, because 98.9% of carbon atoms are 12C, a nucleotide that has no spin. 13C nuclei transition from parallel to antiparallel state is low-energy transition. Because of its abundance in nature is only 1.1%, the sensitivity of the 13C-NMR is much smaller than 1H which has 99.98% abundance in nature.
I think the relationship between biosynthesis, methods of isolation and structural determination of compounds from the natural ingredients are obtained form the compound biosynthesis of natural products that will be made in reference to identify the structure, the structure resulting from the process of biosynthesis of the structure would be similar to the results of the identification or determination of the structure of the compound natural ingredients, and after learning biosynthesis, isolation can do in order to separate the mixed compound so that we can produce a pure single senayawa. From the results of the isolation of a pure single compound we can determine the structure of these compounds using spectroscopic methods with the instruments used are: spectrophotometer ultraviolet (UV), infrared (IR), mass (MS), Nuclear Magnethic resonance (13C-NMR, 1HNMR ).
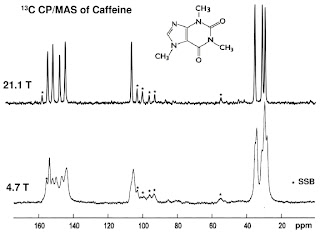
example: caffeine
Biosintesis kafein
Caffeine synthesis in most caffeine producing plants
requires three enzymes to convert Xanthosine to the final product:
Monomethylxanthine methyltransferase (MXMT1), 7-methylxanthosine synthase
(XMT1), and 3,7-dimethylxanthine N-methyltransferase (DXMT1). Both the XMT1 and
DXMT1 genes produce bifunctional enzymes and this particular pathway was chosen
so that one less enzyme would be required in the synthesis (compared to three in
Coffea Arabica)
The
induction of this novel pathway in S. cerevisiae requires two additional
enzymes from C. canephora: XMT1 and DXMT1. S. cerevisiae will produce the
required metabolic precursors to Xanthosine, where after the XMT1 enzyme from
C. canephora will use the substrate to synthesize 7-methylxanthosine, then
7-Methylxanthine. DXMT1 will then convert it to Theobromine and finally
caffeine.
The workings of the isolation of
caffeine from tea leaves
1. 50 grams of tea put in a roll of filter paper, tied with string and insert it into the device sokletasi
2. do the sokletasi with ethanol
3. uapkan solvent by vacuum distillation
4. move as the extract into a cup pengaup containing 25 g of MgO and 200 ml of distilled water
5. uapkan over a water bath while stirring frequently in
6. after a perfectly dry extract 300 ml of distilled water at boil and strain while hot
7. add 25 ml of sulfuric acid 10%, back to volume uapkan a third
8. solution is filtered again to separate heat-deposition
9. did extraction with chloroform to 5 times 15 ml
10. chloroform extracts combine a bit of color, then add a few ml of NaOH 1%, the chloroform layer in the layer with distilled water
11. uapkan chloroform extract resulting viscous residue or extract kofein
12. then do recrystallized by adding distilled water to heat up in pure kofein
13. or do sublimation to obtain pure kofein
caffeine purification by sublimation
caffeine rough (crude coffein) conducted sublimation sublimation using tools made from erlenmeyer accompanied vacuum aspirator vacuum and is equipped with a powered condenser distilled water
characterization of caffeine
1. with reagent mureksid
2. determination of the boiling point
3. with spktrofotometer intramerah
Identification and structure using NMR SpectrumIR
1. 50 grams of tea put in a roll of filter paper, tied with string and insert it into the device sokletasi
2. do the sokletasi with ethanol
3. uapkan solvent by vacuum distillation
4. move as the extract into a cup pengaup containing 25 g of MgO and 200 ml of distilled water
5. uapkan over a water bath while stirring frequently in
6. after a perfectly dry extract 300 ml of distilled water at boil and strain while hot
7. add 25 ml of sulfuric acid 10%, back to volume uapkan a third
8. solution is filtered again to separate heat-deposition
9. did extraction with chloroform to 5 times 15 ml
10. chloroform extracts combine a bit of color, then add a few ml of NaOH 1%, the chloroform layer in the layer with distilled water
11. uapkan chloroform extract resulting viscous residue or extract kofein
12. then do recrystallized by adding distilled water to heat up in pure kofein
13. or do sublimation to obtain pure kofein
caffeine purification by sublimation
caffeine rough (crude coffein) conducted sublimation sublimation using tools made from erlenmeyer accompanied vacuum aspirator vacuum and is equipped with a powered condenser distilled water
characterization of caffeine
1. with reagent mureksid
2. determination of the boiling point
3. with spktrofotometer intramerah
Identification and structure using NMR SpectrumIR